Advanced Diagnostics
& Next Generation Cellular Treatments Coming in 2026
Digital pathology is a modern approach to analyzing tissue samples. It transforms traditional pathology into a more precise and accessible practice. By converting tissue samples into high-resolution digital images, this technology allows for enhanced image quality and analysis. Pathologists can examine these detailed images with greater accuracy, improving the reliability of diagnoses.
Tackling Misdiagnosis in Cancer:
The Future of AI in Digital Pathology:
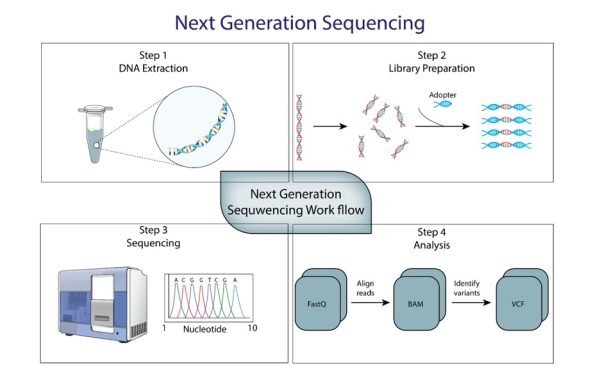
Next Generation Sequencing (NGS) is a transformative technology in modern medicine, fundamentally changing how diseases, especially cancers, are understood, diagnosed, and treated. At the TAM Center, NGS is a crucial component of our diagnostic arsenal, allowing for the rapid and precise decoding of complex genetic information within cancers. This method, which sequences millions of DNA or RNA fragments simultaneously, marks a significant advancement over earlier sequencing techniques by offering a faster, more cost-effective, and comprehensive analysis of genetic material. This leap in technology enables our approach to personalized medicine, where treatments are tailored to the individual’s genetic profile, with the potential to enhance effectiveness and reduce adverse effects.
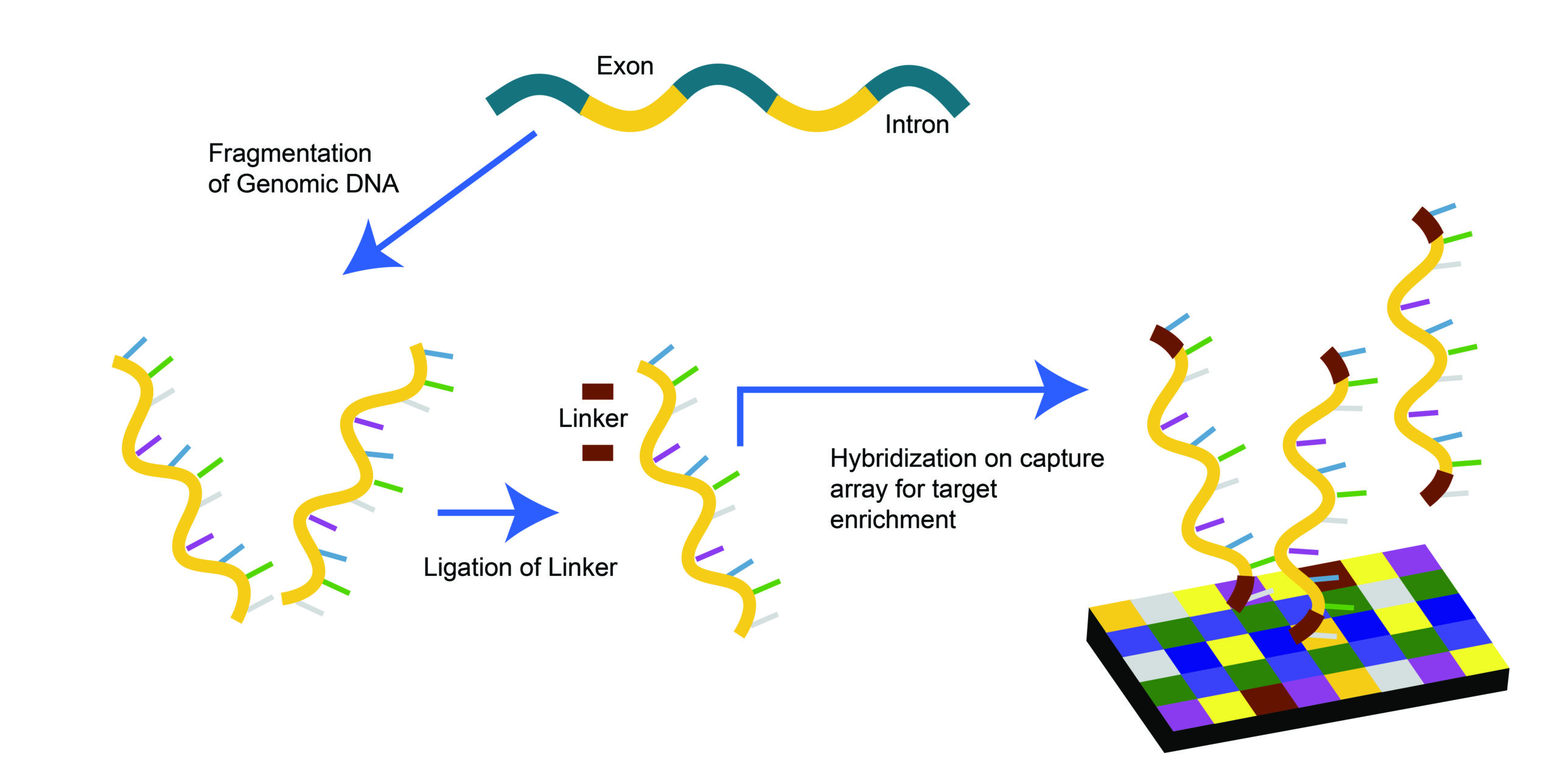
Whole Exome Sequencing (WES) for tumors represents a pivotal advancement in our understanding and treatment of cancer at the TAM Center. This technique focuses on sequencing the exome, which consists of all the genome’s exons; the coding regions of genes that are translated into protein. Since the majority of known genetic mutations associated with diseases are found in the exome, WES offers a targeted approach to uncover the genetic underpinnings of cancer and provides valuable insights into neoantigenic targets for personalized cancer therapies.
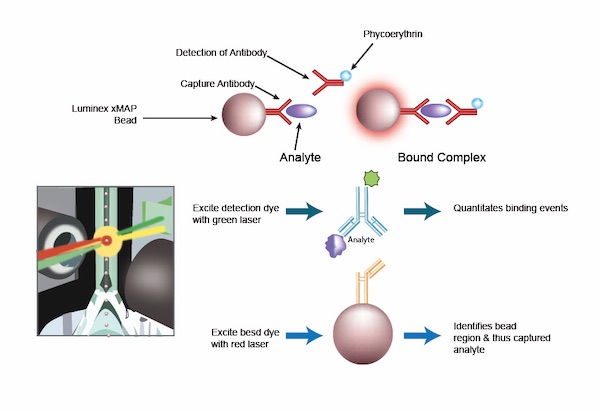
Proteomics is the study of the full set of proteins produced by a genome, cell, tissue, or organism, and is another cornerstone of our approach at TAM Center. This detailed analysis of proteins provides invaluable insights into the biological processes and mechanisms of diseases, including cancer. We implement specific Luminex assays for assessing how patients are responding to treatment.
Bioinformatics stands at the crossroads of biology and computational science, providing the tools necessary to navigate the complex data generated by modern genomic research. Since its emergence in the 1960s, and particularly after the milestones achieved by the Human Genome Project, bioinformatics has been indispensable in transforming raw molecular data into comprehensive insights. This discipline focuses on the analysis of genomic sequences, protein structures, and their functions, making sense of the vast quantities of data that define the building blocks of life. In the context of cancer treatment, bioinformatics enables us to understand the genetic aberrations that drive the disease, paving the way for targeted therapeutic strategies.
Interventional Radiology (IR) represents a pivotal advancement in the diagnostic and therapeutic landscape of medicine, particularly in oncology. The field has evolved significantly since the first successful angiograph in 1923 and was further revolutionized in 1953 by Swedish doctor Sven Ivar Seldinger with the introduction of the Seldinger Technique. This innovation laid the foundation for Charles Dotter, often referred to as the “Father of Interventional Radiology,” to further develop the field in 1963. Today, IR leverages minimally invasive imaging techniques to diagnose and treat various conditions, including cancer, with unparalleled precision.
Cryoablation represents a sophisticated method in the arsenal against cancer, employing extreme cold to selectively destroy tumor cells. This minimally invasive procedure is guided by advanced imaging techniques, such as Computed Tomography (CT) and Ultrasound, ensuring precision and effectiveness in targeting the tumor while minimizing damage to surrounding healthy tissues.
The Role of CT in Guided Cryoablation
The Advantages of Ultrasound in Cryoablation
The Synergy of CT and Ultrasound-Guided Cryoablation
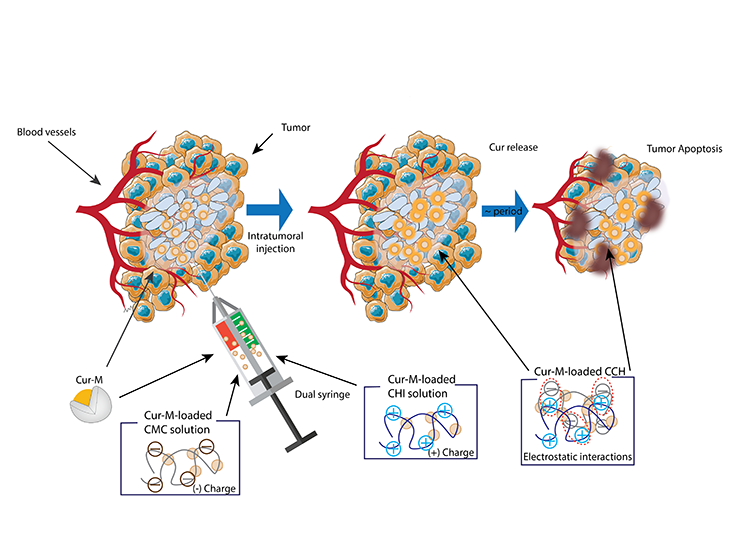
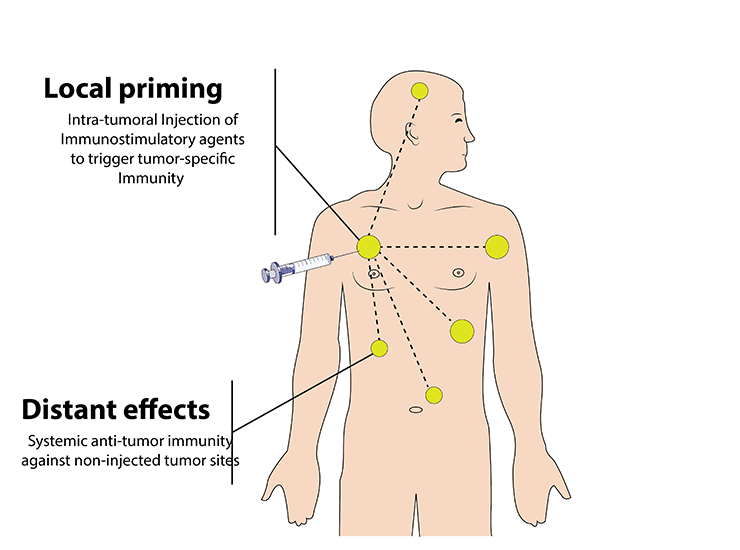
Intra-tumoral immunotherapy injections represent a targeted approach to cancer treatment, leveraging the body’s immune system to fight tumor cells directly at the site. Administered during a patient’s stay at the hospital, these injections are precisely guided by ultrasound or CT imaging, ensuring that the therapeutic agents are delivered directly into the tumor. This method stands out for its specificity, aiming to elicit a tumor-specific immune response through the direct application of immunotherapeutic agents.
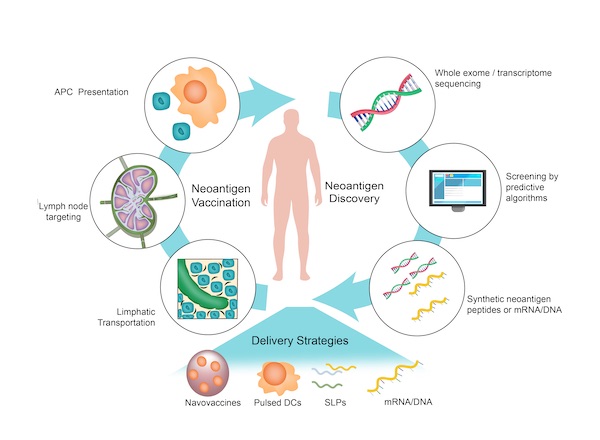
Neoantigen vaccines stand at the vanguard of personalized cancer treatment, leveraging the unique genetic alterations of an individual’s tumor to elicit a targeted immune response. This cutting-edge approach to immunotherapy is profoundly dependent on the integrated workflow of our bioinformatics and cancer care team, incorporating Whole Exome Sequencing (WES) and Human Leukocyte Antigen (HLA) matching, which together enable the precise identification and utilization of neoantigens for personalized vaccine development.
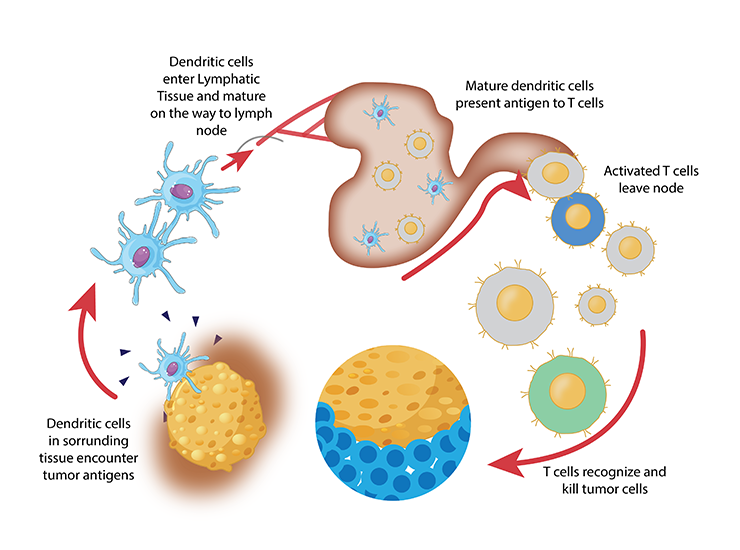
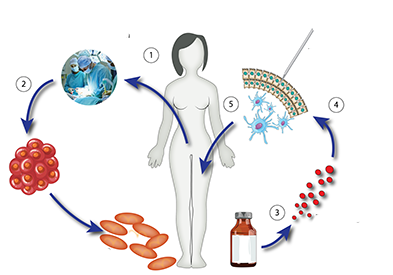
At TAM Center, we have been at the forefront of utilizing autologous dendritic cell (DC) vaccines for over two decades, a testament to our commitment to pioneering cancer immunotherapy. Autologous DC vaccines are a personalized form of treatment that leverages the body’s own immune cells to mount a targeted attack against cancer. These vaccines employ dendritic cells, key players in the immune system known for their ability to present antigens and activate T-cells, thereby bridging the innate and adaptive branches of the immune system.
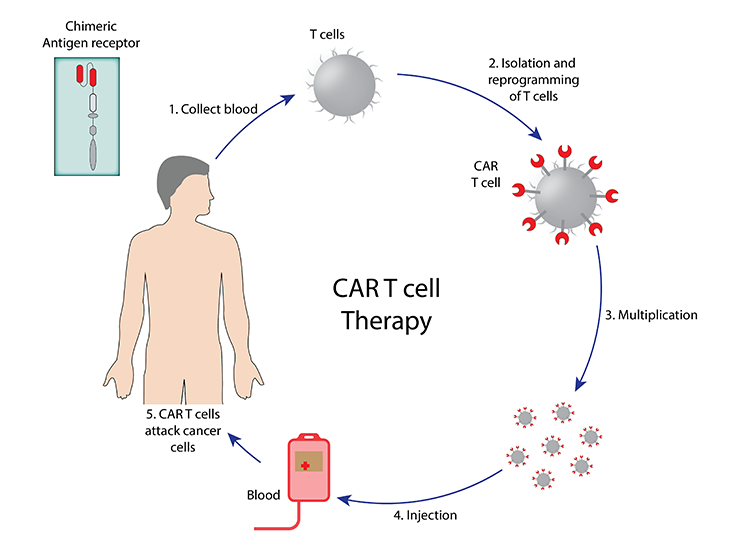
CAR T-cell therapy represents a groundbreaking approach in cancer treatment, particularly for certain types of blood cancers. This innovative therapy involves reprogramming a patient’s own T-cells-a type of immune cell-to recognize and attack cancer cells.
How CAR T-Cell Therapy Works:
Examples of FDA-Approved CAR T-Cell Therapies:
The Future of CAR T-Cell Therapy:
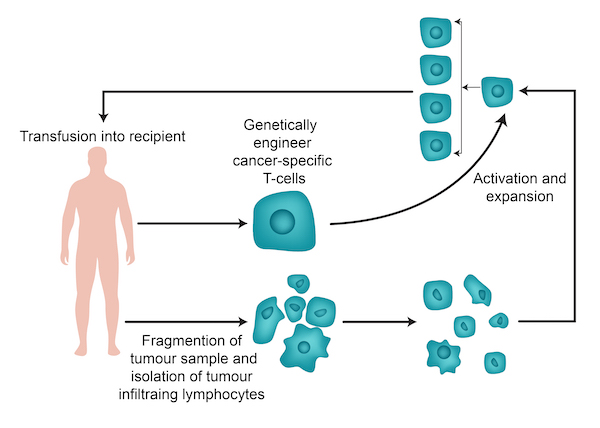
Tumor Infiltrating Lymphocytes (TILs) are a pivotal aspect of the immune system’s arsenal against cancer, offering a promising avenue for treating various solid tumors. The utilization of TILs in cancer therapy has been significantly advanced by pioneering work conducted by Dr. Steve Rosenberg and his team at the National Cancer Institute (NCI), laying the groundwork for contemporary immunotherapy approaches.
TIL Therapy: Process and Mechanism
Clinical Evidence of Efficacy
Future Directions and Expanding Applications
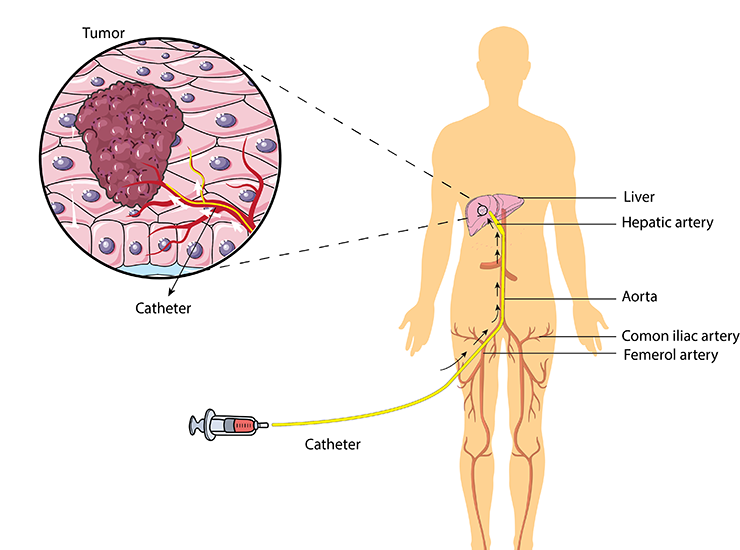
Transarterial Chemoembolization (TACE) is a targeted cancer treatment performed by our skilled interventional radiology team. This procedure involves delivering chemotherapy drugs directly into the arteries that supply blood to the tumor, offering a focused approach to treatment.
How TACE is Performed:
Applications of TACE:
Additional Uses and Considerations:
At the heart of the Translational and Advanced Medical (TAM) Center’s innovative approach to healthcare is our collaboration with the Cellular Performance Institute (CPI), a pioneering force in the field of stem cell therapy. CPI, located on the 5th floor of the TAM Center, is recognized as a world leader in leveraging stem cell treatments for musculoskeletal conditions, showcasing a commitment to advancing regenerative medicine.
Hypoxic Mesenchymal Stem Cells (MSCs)
Advantages of Hypoxic MSCs:

Heart disease remains the leading cause of death in the United States, claiming approximately 700,000 lives each year. This staggering statistic underscores the urgent need for innovative treatments and interventions that can effectively address the myriad complexities associated with cardiovascular conditions. At the Translational and Advanced Medical (TAM) Center, we are committed to confronting this challenge head-on by exploring and implementing cutting-edge therapies that hold the potential to revolutionize cardiac care.
The Procedure Explained:
High-Tech Facilities and Expert Care:
Chronic Obstructive Pulmonary Disease (COPD) is a progressive and debilitating condition that affects millions worldwide, characterized by increasing breathlessness, frequent coughing, wheezing, and tightness in the chest. Historically, COPD has been associated with long-term exposure to harmful particulates or gases, most commonly from tobacco smoke, occupational dust, and air pollution. Over time, COPD leads to a gradual loss of lung function, making everyday activities increasingly difficult.
Follow The New TAM Center
TAM Center is undergoing upgrades from top to bottom as we further our commitment to the very best in cancer care.